Myelofibrosis Treatment
Dr. Srdan Verstovsek
March 2022
Srdan Verstovsek, MD, PhD of MD Anderson gives a detailed overview of latest research involving myelofibrosis treatments, with a highlight on the recently approved MF drug pacritinib.
- Myelofibrosis Treatment
- What are the hot areas in blood cancer treatments?
- What is your philosophy when it comes to drug combinations?
- The impact of anemia on stopping treatment
- Pelabresib
- How soon would patients might have access to both pelabresib and luspatercept?
- Pacritinib overview
- FDA accelerated approval in myelofibrosis treatment for underserved patients
- Is there talk of cure for myelofibrosis?
- The Prognostic Scoring System
- More insight from Srdan Verstovsek, MD, PhD
Myelofibrosis Treatment
The Patient Story: Dr. Verstovsek, you were instrumental in helping to develop ruxolitinib, which is sort of the gold standard for a long time now. So we’ll be talking about today about different combinations and also novel therapies.
We’ll start with myelofibrosis as a category because I understand that what’s research there often can be then may be applied for the other areas, which is really promising.
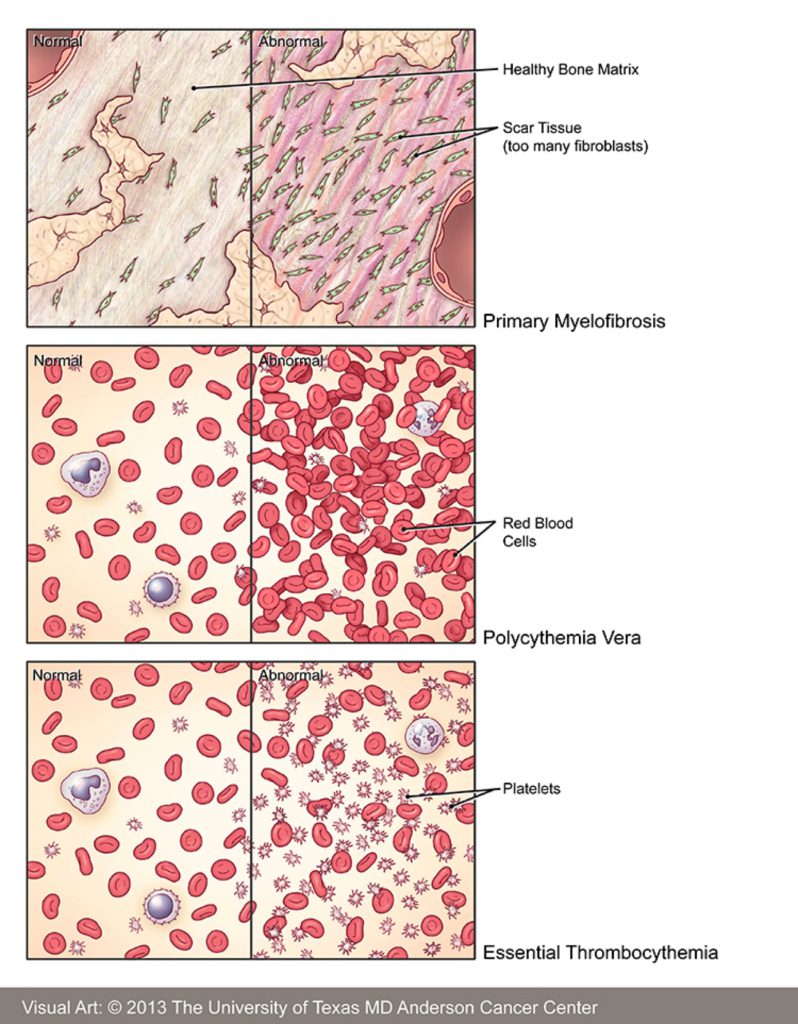
Basics of myelofibrosis
Dr. Srdan Verstovsek: Let’s understand what’s happening with the therapy for patients.
Myelofibrosis. The main three reasons why we treat these patients is to control what bothers them the most:
- That is enlargement in the spleen. 80% of the patients will have a very big spleen, 40% will have a very big liver. So imagine 20 times the normal size of the big organs.
We don’t have a cure. We have medications to control the signs and symptoms.
- At the same time, a lot of systemic symptoms:
- night sweating
- low-grade fevers
- itching
- bone aches
- pains
- body wasting, inability to walk
- decreased performance status: fatigue, weakness.
- Number three, the bone marrow doesn’t work anymore.
Anemia is a major problem. The spleen symptoms and anemia is the major reason why to treat the patients.
Ruxolitinib
We don’t have a cure. We have medications to control the signs and symptoms. The ruxolitinib that you mentioned, it’s now been about 10 years since approval as a standard practice to control the symptoms and the spleen.
But it can worsen the anemia, and there are limitations to what it can do. The median duration of therapy seems to be several years, maybe three years, maybe a little longer, maybe a little shorter. But the issue is how we can improve that.
Pelabresib
Pelabresib, the drug that you are talking about, is a new investigational medication that is being combined with the ruxolitinib from day one of the therapy to improve – the ruxolitinib does more of the spleen control. Make it smaller – as small as possible in promoting quality of life, eliminating in the majority of the patients as many as you can.
No night sweat, no fever, no weight loss, improve the body composition, make people feel normal and appear normal and possibly in addition, decrease that anemia that is problematic.
Remember, with the ruxolitinib, you can control the spleen and symptoms, but you cause anemia. So adding pelabresib, maybe you can tackle all three together. The same drug is being studied in different case scenarios, like after ruxolitinib as a single agent.
So there are many different roles in how you can develop different drugs in different scenarios of patients who have different needs. They are presented in a different way, but a combination from day one appears to be very promising.
This drug, by the way, improves bone marrow function by altering genetic expression. The genes that carry the message in the bone marrow. What the cells should do is be altered by these medications for the better, and that should improve the bone marrow function and work.
Together with the ruxolitinib, ruxolitinib inhibits the growth of the cells and inhibits the inflammation that comes with the myelofibrosis. That’s why people feel better, and that’s why the spleen is smaller. So, the two together may be working, and that is underway. A Phase 3 study means that something is compared to old-fashioned therapy. Something new to old.
Once we confirm that two are better than ruxolitinib alone, we’ll see.
The two together appear to work better than what you would expect from ruxolitinib alone. That’s what we have so far. We have what we call phase two to study. Everybody gets the therapy. Everybody is uniformly treated. We have very good results. A great majority of people decrease the spleen, decrease the symptoms. Maybe even improve the anemia, as I said before.
You want to confirm that this is true. Now you have to randomize patients between the single-agent ruxolitinib and the combination. That’s underway already as we were learning more about excellent results of the combination in this Phase 2 study. The Phase 3 study has been already initiated globally to confirm. And maybe the two together will become new standard practice in two or three years. Once we confirm that two are better than ruxolitinib alone, we’ll see.
What are the hot areas in blood cancer treatments?
The Patient Story: We hear in different blood cancers, the shifting of adding another and seeing how drugs do in combination. Is this one of the hot areas?
Dr. Srdan Verstovsek: This is a very hot area. So if we described what happens in community practice, it’s just ruxolitinib alone. You may envision then the combinations to be better from day one.
And in addition to pelabresib in combination with ruxolitinib, there are a couple of other medications that are being tested for the same purpose from day one, with other medications that tackle other biological abnormalities in the bone marrow myelofibrosis cells.
So you actually have at the moment, not just collaboration and two others of phase three randomized study underway globally kind of competing for each other. But this is good. You have three drugs that are trying to be combined with the ruxolitinib to say. hey, we are better than the ruxolitinib alone.
You can also envision that some patients just ruxolitinib alone for some time. They may have a suboptimal response, or patients are losing response slowly. That typically means the spleen is growing back, the anemia is slowly developing, or the symptoms are coming back.
This is, after all, a chronic disease. Nothing really changed overnight. It takes time. So we call this like suboptimal responders or loss of response. People are still on ruxolitinib, and that’s the second way of combinations adding another drug to people who are already on this standard therapy but not optimally managed or they have problems with it. That’s a different way of combinations.
There are four different Phase 3 studies in this way where people are all already on the therapy, responding to some degree, you’re adding in something, add on approach—many studies. I encourage everybody to look it up online. It’s all online, and try to find the studies to participate in.
What is your philosophy when it comes to drug combinations?
The Patient Story: It could be beneficial for them, and then, of course, it’s beneficial for research and making sure we get to those new drugs. And so I have to ask you – there are some other areas where people talk about, well, we should start off if we can determine that a combination is really great and more effective that we would, then, just go right off the bat. We don’t have a crystal ball, but what is your philosophy when it comes to this so far?
Dr. Srdan Verstovsek: Yes, it’s a mixed bag, because if you are saying we should combine things together from day one to get better control discipline and better control, the symptoms. That all makes sense, and earlier intervention with the best you have will make the best impact on the patient’s overall outcome.
But until proven, some doubt and say, well, maybe I just start with the standard one pill, and if I’m not doing well with that one pill, then I’m going to add another one. Maybe not everybody requires the two pills from Day One. It all makes sense.
Different combinations for different purposes
But some of these combinations are really looking at some different angles of what the problem is. For example, we talked so far about enhancing the spleen and symptom control, but there is a combination with another medication, which is called luspartecept. It’s a difficult one. It doesn’t need to be remembered.
Luspartecept + ruxolitinib
But that particular medication is being combined with the ruxolitinib for only one purpose, to improve the anemia. So it’s not really to enhance what the ruxolitinib JAK inhibitor does. No more of the spleen and more of the symptoms, but make it easier because instead of expecting anemia to happen through you, the ruxolitinib, you improve the anemia. The two together then cover very well all the three problems: the spleen, the symptoms and anemia.
This is a medication that is in Phase 3 study in people who are on ruxolitinib but have a significant anemia. So it’s one of these add-on approaches when it’s necessary.
The alternative view of the problem there was a medication called sotatercept presented in public. Sotatercept, luspatercept you see similar names there in the same branch of the drugs – anemia, drugs. We call them anemia drugs. They are injectable under the skin every three weeks and no side effects that much whatsoever. Very safe.
Luspatercept is already approved for a different condition of the bone marrow as an anemia drug for a condition called myelodysplastic syndrome. And it is being studied as sotatercept for patients with myelofibrosis anemia.
A year ago, we had a presentation on luspatercept. No other drug works make it easier to manage version with JAK inhibitor.
This year we had a presentation on sotatercept as an add-on to people who are on ruxolitinib, and about a third of the patient’s anemia significantly improves. So people feel better, no transfusions anymore. They are on ruxolitinib. They have a smaller spleen, a better quality of life; anemia is not a big problem anymore. That particular combination is very attractive to many. It’s a clear-cut message of what it is for anemia.
No other drug works make it easier to manage version with the JAK inhibitor. The sotatercept-luspatercept very enthusiastic crowd out there for anemia drug to be developed. Some patients are very anemic on their own. They don’t have a big spleen or symptoms. Not everybody is the same in myelofibrosis.
So what do we do if the patient comes in with bad anemia and there is no big spleen or symptoms? Well, you don’t have much to offer. There is no approved anemia drug. So there is a lot of enthusiasm and potential for anemia drugs in myelofibrosis.
The impact of anemia on stopping treatment
The Patient Story: This is really important because sometimes the anemia is so bad it can badly impact the ability to continue on treatment – is that what this is ultimately addressing?
Dr. Srdan Verstovsek: You are absolutely correct. The main reason why people eventually stop ruxolitinib is anemia. Anemia can be caused by the therapy. It can be present already from the beginning through the disease process. The ruxolitinib being a JAK inhibitor, inhibits the growth of the cells and inflammation, so there are benefits. But the disease is not eliminated. So, elimination is not possible.
So, disease can’t change over time. You improve the spleen and symptoms, but the disease will change. Perhaps new abnormalities will be acquired during the therapy as any other disease does acquire new problems, and anemia develops over time.
Then you have to stop the ruxolitinib, and you look for something else. So, if you can counteract the anemia being the main problem for discontinuation of ruxolitinib, that’s going to be a big deal.
Pelabresib
The Patient Story: If we rewind just for one moment, pelabresib, and you were talking about side effects, can you just describe some of the more common ones that have been seen?
Dr. Srdan Verstovsek: Ok, so pelabresib is a pill and it’s given daily for two weeks and then one week off. It is given this way because in earlier studies, not in myelofibrosis, but in other studies before it came to myelofibrosis, it showed that it could lower the platelets. By doing this two weeks on, one week off, it does not appear to be causing a major issue with the platelets.
Pelabresib side effects
- GI tract low-grade irritations:
- nausea
- vomiting
- diarrhea
There are some other low-grade irritations of the GI tract, like nausea, vomiting, or diarrhea. They do not appear to be common at all, and in fact, people don’t really complain much. You can give patients some anti-nausea and anti-diarrhea medications. Being mild on the symptoms, not really causing the major problem and not causing the anemia and perhaps improving the anemia from these ongoing studies.
The balance between efficacy and safety is much so on the benefit of efficacy. And that particular constellation of the balance makes it so favorable to be combined with a ruxolitinib. It’s not causing any new toxicities; it’s not providing the toxicities ruxolitinib does. So there is no combination of the toxicities that would hurt the patient. Overall, the safety profile is very much in favor of the combination.
How soon would patients might have access to both pelabresib and luspatercept?
The Patient Story: And I know that’s a big question for a lot of people who are thinking about if this comes down the pipeline, just to wrap for those two, for both pelabresib and sotatercept. How soon would patients approximately think that they might have access to it?
Dr. Srdan Verstovsek: Luspatercept, instead of sotatercept, is the one who is the Phase 3 study for approval as an anemia drug in myelofibrosis. That’s a Phase 3 ongoing study and the pelabresib that we said also Phase 3 ongoing study for possible approval as a combination partner from the very beginning to enhance the spleen and the symptoms response and be mild on the blood cell count not to cause too much suppression. Both Phase 3 studies are underway.
Usually it takes a couple of years for them to really accrue all the patients. We are talking about several hundred patients around the globe. The patients need to be followed, every single patient, for at least six months. Remember, none of this is chemotherapy. They are biological agents.
The ruxolitinib being JAK inhibitors, inhibition of the proteins inside the cells. We talk about pelabresib expression of the genes in the bone marrow cells, luspatercept and sotatercept enhance the maturation of the red blood cells. So less anemia. Red blood cells are the ones that carry oxygen around. So, in that setting, it takes time.
Six months is the standard practice to assess the benefit, no less. And so if you have three or 400 patients to accrue and every single patient follows for six months. Well, maybe two or three years to maturation and to know whether they are good enough for approval.
Pacritinib overview
Editor’s note: By time of publication, FDA approved pacritinib or Vonjo for myelofibrosis patients and severe thrombocytopenia.
The Patient Story: Ok, great to know, and of course, in the meantime, if anyone needs that access, if they can try to apply to be on a clinical trial, it’s probably the best way.
So as we shift then to the pacritinib and address the studies on the safety and efficacy of that. But we’re talking about a population that has moderate to severe thrombocytopenia, so this is addressing a whole another subset of people who might have a lower dosage on ruxolitinib because they can’t handle it. Could you give us more of an overview and the latest on that one?
Dr. Srdan Verstovsek: The ruxolitinib is for myelofibrosis patients to control the spleen and symptoms, but it lowers the blood cell count. We talk about anemia as the major problem.
The ruxolitinib is not even suggested to be given in patients with low platelets below 50,000 platelets, and even between 50,000 and 100,000, it’s to be given at a low dose. The normal (number of platelets) is between 150,000 and 450,000. So we are talking about the decreased platelets.
Fedratinib
By the way, about two years ago another JAK inhibitor called fedratinib was approved with the same notions, but it cannot be given to patients with low platelets below 50,000 because these two drugs potentially can worsen that. And then there is the risk of bleeding.
Pacritinib, the one that you are mentioning, is another JAK inhibitor, another inhibition of this JAK enzyme inside the bone marrow cells that are important for proliferation and inflammation. So it also improves the spleen and symptoms. But it’s not. That’s the key difference. It’s not lowering the blood cell count.
It is being developed as a drug for patients with platelets below 50, maybe even below 100. But below 50 is the critical group of people because you can’t really give them much at all. So far, in the multiple different studies, it was a pretty effective drug.
FDA accelerated approval in myelofibrosis treatment for underserved patients
The Patient Story: Right. You’re talking about when there’s an urgent need, and there’s a population that doesn’t even have options. The FDA, there’s an accelerated approval process, and so we’ll cross our fingers and hope for the best there. And is this the first line?
Dr. Srdan Verstovsek: Because right now, there is nothing you can do for patients with platelets below 50. You’re not really serving very well. The patients with platelets between 50 and 100 but below 50 are the critical point. So any patient’s front line out from per day, the platelets are so low you can’t really do much with current medications. Pacritinib will definitely have a role as a number one choice in these people.
I don’t tell my patients how long they’re going to live. I’m telling them the truth that I’m going to try to get that life as long as possible.
Is there talk of cure for myelofibrosis?
The Patient Story: Wonderful. As we wrap this section focused on MF, I do want to ask, we talk about treatments, and you are saying very at the start of this conversation that, when you started, it was palliative care. It was hospice. There was nothing.
Then it shifted and then there was ruxolitinib. There’s been a lot in terms of controlling, whether it’s the spleen, symptoms, anemia – when are we going to be able to shift to something even beyond that?
Dr. Srdan Verstovsek: Yeah. You see, we are first addressing the immediate need of the patients. You want to make people feel better. You want to decrease the big organs. You want to make them functional, participating in society fully in family life for as long as possible.
And then you want to improve the bone marrow function, improve the anemia. You want to prevent progression. You want to maintain this as long as possible. These are the current studies that enhance what you do for as long as possible because I always tell you there is an expiration date of any medication. So you have to improve its combinations.
As we said, maybe additional medications like in a second line after the first ruxolitinib or fedratinib or pacritinib, but there is another drug later on. We have one study so far which looks at the prolongation of life as the endpoint or approval.
We are ready with these new approaches not to go into detail, but maybe some of these combinations will enhance what we do, and we’re going to say we’re going to prolong life. So I don’t tell my patients how long they’re going to live. I’m telling them the truth that I’m going to try to get that life as long as possible.
We already have a very good knowing that with the really good management of the patients with optimal dose in combinations, perhaps with the novel or all drugs when they’re combined in a different way, we can make people live longer.
We can eliminate the disease. No cure, unfortunately. But it seems from many sources with the ruxolitinib, particularly people believe three to five years longer from retrospective or prospective.
So looking in the past or looking at what’s happening right now as we go through life, there are multiple sources of information that suggest that over the last 10 years, the outcome of the patients with more fibrosis has significantly improved. Three to five years longer life expectancy. So that is the building block, whereas to say if we cannot cure it, we can make it as chronic as chronically possible so that we don’t read in textbooks anymore.
But you still read three to five years on average survival – that’s from 10 or 20 years ago. So I don’t tell my patients how long they’re going to live. I’m telling them the truth that I’m going to try to get that life as long as possible. With the good quality of life, and I can’t really say how long it is, it’s going to be long.
The Prognostic Scoring System
The Patient Story: I really appreciate that that was actually my last question, which was going to be because you’re such a part of so many of these trials, and you see the fresh data that, yeah, a lot of the numbers that patients and caregivers are reading and are told are from a long time ago, and it can be quite daunting. And so the question was, and you’ve answered it. So it’s adding already three to five years. And of course, this is high-level broad brushstrokes. So we’re not talking about one particular patient. It’s different for everybody, but on the whole, it’s trending in such a positive direction.
That’s why I apply that prognostic wisdom for only one purpose: to refer the patients to the bone marrow transplant.
Dr. Srdan Verstovsek: Let me add a little bit here on the prognostic scoring systems. It is typical in cancer and some other conditions that you would say, let me see whether there are any patient characteristics that would tell me what the future. And so you look back 20, 30 years ago and you see maybe 500 patients or whatever the number is that you can put together and you analyze their outcome and you come up with some prognostic factors that made some people live longer.
Some people live longer, shorter, and you come up with, let’s say, five of them, and you put them together and said, this is my prognostic scoring system. I put these five factors and say, What’s the future for the patient that I see now? But the point I’m making is that from 20 years ago.
So with myelofibrosis, in particular, I am not in favor of such an approach because life is different and therapies are different right now. That’s why I apply that prognostic wisdom for only one purpose: to refer the patients to the bone marrow transplant.
The addict might have really a shorter life expectancy than five years because you want to refer that patient to a bone marrow transplant. Bone marrow transplant is the only curative potential we have, and it’s justifiable because there is some death associated with the procedure. Be honest. it’s not that easy.
People die from transplants. You have to be careful who goes for a transplant, and we say if the life expectancy and the only way that you can do that is really to apply those prognostic scoring systems, but they are relative. As you see, if we say that yes, life expectancy is possibly shorter, you should do the transplant.
That’s why I apply that prognostic wisdom for only one purpose to refer the patients to the bone marrow transplant. And of course, then the transplant doctor will have their own prognostic system for the outcome of the transplant, which is completely different.
More insight from Srdan Verstovsek, MD, PhD
Myeloid/Lymphoid Neoplasm Breakthrough (2022)
Dr. Verstovsek describes one of the most exciting drug developments has happened for MLN patients.
Polycythemia Vera (2022)
In segment 2 of our conversation with Srdan Vertsovsek, MD, PhD of MD Anderson, he shares the latest on research for polycythemia vera or PV treatments.
Myelofibrosis Treatment Options (March 2022)
Dr. Verstovsek gives a detailed overview of latest research involving myelofibrosis treatments, with a highlight on the recently approved MF drug pacritinib.



