CAR T Cell Therapy in Multiple Myeloma
A Conversation with Rafael Fonseca, MD
Published August 2021
Dr. Fonseca describes emerging T-cell therapies, describing the role T-cells play in our bodies and then focusing on chimeric antigen receptor (CAR) T-cell therapy and bispecific T-cell engagers.
The interview has been edited only for clarity.
Background on the role T-cells play
One of the areas where we have the most excitement is this whole field of CAR T-cells and the bispecifics. For background, we all have T-cells in our bodies. Most of the cells that circulate in our blood that belong to the family are called the lymphocytes.
For patients who know about this, you know that we measure the neutrophils. It’s the most common one, especially if you want to transplant. You know very well what the neutrophils are or the so-called ANC (absolute neutrophil count).
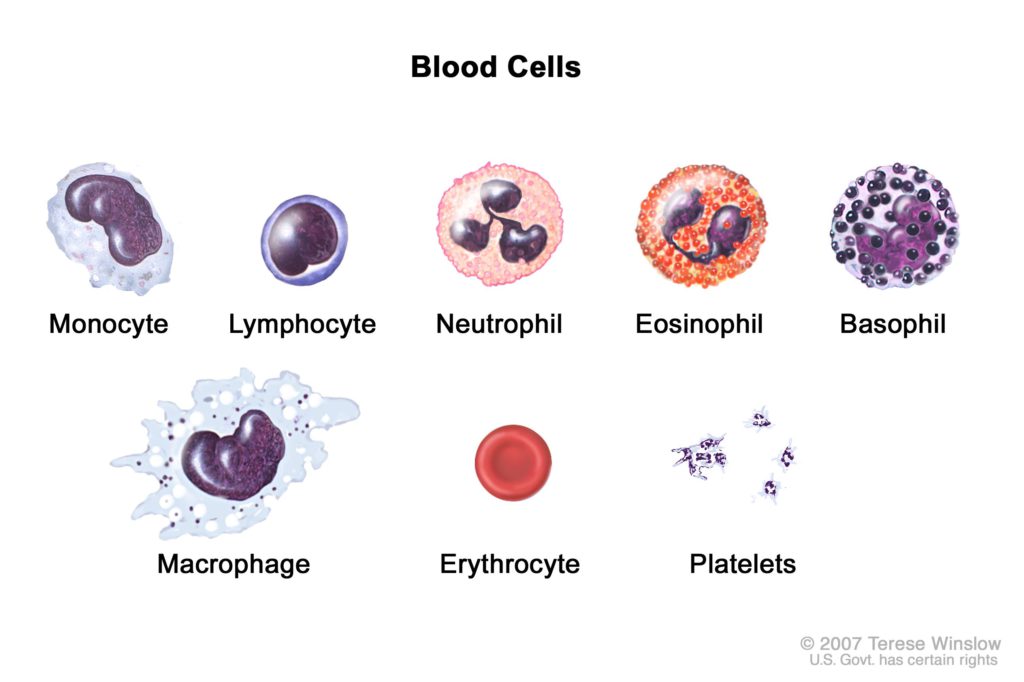
If you look at the blood counts, the next one you’ll see is lymphocytes. Most of those lymphocytes, at least 70% to 80% of those lymphocytes belong to this family. They’re called the T lymphocytes, so the T-cells or the T lymphocytes.
Now these cells are natural bullies. They are just total bullies. What happens in our body if T cells go around, you want to get out of its way because if a T-cell latches onto something, it just starts attacking.
It’s looking for areas where it can help. They help the human body fight off certain infections and protect us against tumors. That’s the main role of T-cells.
They’re part of our immunity. We even have heard about this with COVID. Someone came up with the idea of saying, “Well, if they are bullies, what if we make them go after myeloma cells? What if we prime them to go after myeloma cells?”
With the advent of genetic engineering, people thought, “Well, we can take them out of the body. We’ll reprogram them so they have anchors that match those that exist in the myeloma cells. Then we’ll give them back into the body.”
CAR T Cell Therapy in Multiple Myeloma
15 years ago, this was science fiction. People were not too convinced about immunotherapy back then. It turns out it works.
If you give back T-cells to someone, they go in, they get infused, they grow, they expand. They divide and they start forming a rebellion against myeloma cells. Those are what we call CAR T-cells. The process takes a few weeks.
A person who’s a candidate, you collect their T-cells, they’re sent to a facility where they are processed, then they’re infused back into the person, and they do their thing. It’s usually a one-off thing.
I use the analogy of that fable of the frog that has been asked by the scorpion to cross the river, and then ultimately convinced it. As soon as the scorpion is on top it stings. That’s the nature of T-cells. T-cells do that, like the scorpion.
Bispecific T-cell engagers (bispecifics)
That’s all good and well, but then someone else said, well, if we get the T-cells out, what if we, instead of getting the T-cells out, infuse something that works like a matchmaker. Something that brings together T-cells and myeloma cells.
There are happy T-cells circulating there. If we put them next to myeloma cells, we know the T-cells are bullies, they’re going to go after them.
That’s what the matchmaker does, what we call bispecific antibodies. These are antibodies that are on one arm, they attach to the T-cell and the other arm, they attach to the target cell, in this case, myeloma.
I describe them like double-sided Velcro. One of the Velcros attaches to the myeloma, the other one to the T-cell. It turns out that we have several of them that are highly effective. I believe we had about seven of them that were presented at the ASH meeting. The idea, the potential advantage over CAR T-cells, is that they’re off the shelf.
You get to the clinic, you need treatment. You mix the antibody and you infuse it in the person without having to wait for that engineering process.
Just hang on to your seats, that will be a battle that will be happening over the next several years as to what is better, the CAR T-cells or the bispecifics.
But ultimately this is for the good of patients because this is going to be one of the ways in which we treat myeloma.
Weighing between CAR T and BiTE
- CAR T-cell therapy
- Pros: Great track record, one-time treatment (typically)
- Cons: Takes longer to complete (cell collection, sent to specialized lab), must undergo chemotherapy, could have myelosuppression
- Bispecific T-cell engagers
- Pros: “Off the shelf” (ready to go)
- Cons: Short track record, requires repeated administration
The reported pros of CAR T-cells are that they have a greater track record, and because of their ability to expand and their ability to persist, they could be a one-off treatment that does it.
In fact, we have long standing responses and histories. There was that patient treated at UPenn, for whom I think they did a recent story in The New York Times, as well. Just someone who has had essentially a cure because of the CAR T-cells.
I think that’s the major plus – that it has a track record and it’s somewhat specific. People are very excited with that.
The downside is that you have to collect the T-cells and they have to be sent somewhere. It takes a few weeks and then they come back. And in the meantime, you have to give more chemotherapy.
Even when they come back, a person can have a period where you have the blood counts that are low, what we call myelosuppression. That increases somewhat the complexity, and creates certain infection risk, and other things that one has to be mindful about. There’s a recovery period.
The bispecifics on the other hand, as I mentioned, they’re off the shelf.
You could imagine there’s a pharmacy, and it could be in that community hospital, doesn’t have to be a specialized hospital. I think people are rightfully concerned that they want to see more follow up of the depth and the duration of those responses.
The other one is that they’re required repeated administration. It’s not going to happen in a one-off, it’s something that we’re required – repeated treatment. That’s another disincentive. I think whichever way it works out, whatever is the winner, it will be embraced by patients.
There may be more than one winner, which is great news because with the bispecifics, not all of them target BCMA. Some of them target other molecules, GPRC5D, FcRH5. As you’ve noticed, I have to study all of those.
Those are all new targets for some of these antibodies, like SLAMF7. So maybe someone fails the bispecific, but can go through a CAR T-cell or vice versa.
How soon can we see bispecifics being used on patients
I don’t know. It’s a great question. We just had our first CAR T-cell approved this year. Hopefully the next one, pretty soon. We just can’t get enough production capacity.
It’s really just gut wrenching right now because we have waiting lists and just people who really want to get into this.
We’re opening as many clinical trials as we can, so we have this available for patients. The bispecifics are still somewhat early on and it depends on what level of evidence is required by the regulators.
My sincere hope would be that they are available by next year, by 2022. Some of those trials are already treating hundreds of patients. We have precedents for this with other treatments in myeloma.
It would sound just almost science fiction, but it’s possible that in 2022, we would have two CAR T-cells and three antibodies that are going to be available for the treatment of patients.
